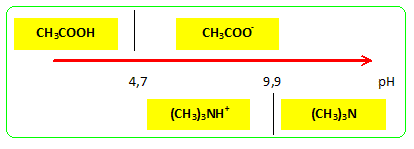
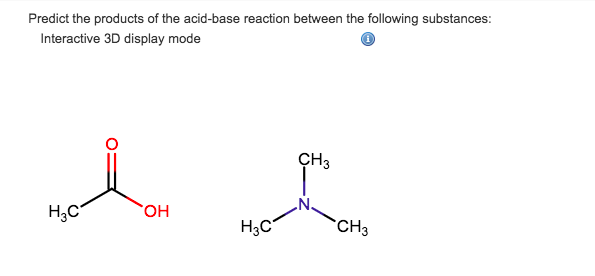
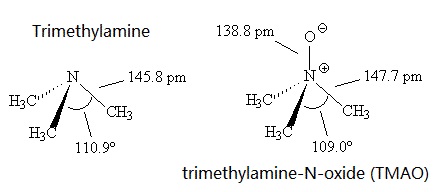
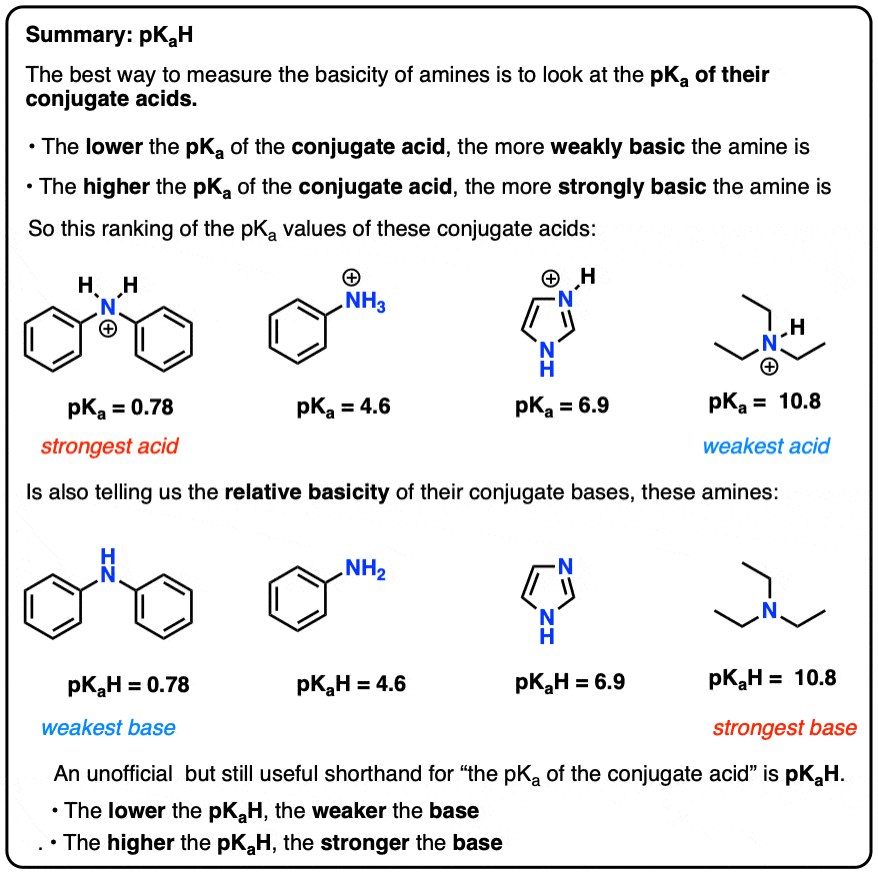
Welcome to Chem Zipper.com......: Why trimethylamine amine ( N(CH3)3) is tetrahedral while trisilyl amine (N(SiH3)3) planner.?

Pls explain: Q Trisilyl amine is a weaker base than trimethyl amine because (a) in trisilyl amine pπ-pπ bonding - Chemistry - The p-Block Elements - 12586833 | Meritnation.com

mass spectrum of N,N-dimethylmethanamine (trimethylamine) C3H9N (CH3)3N fragmentation pattern of m/z m/e

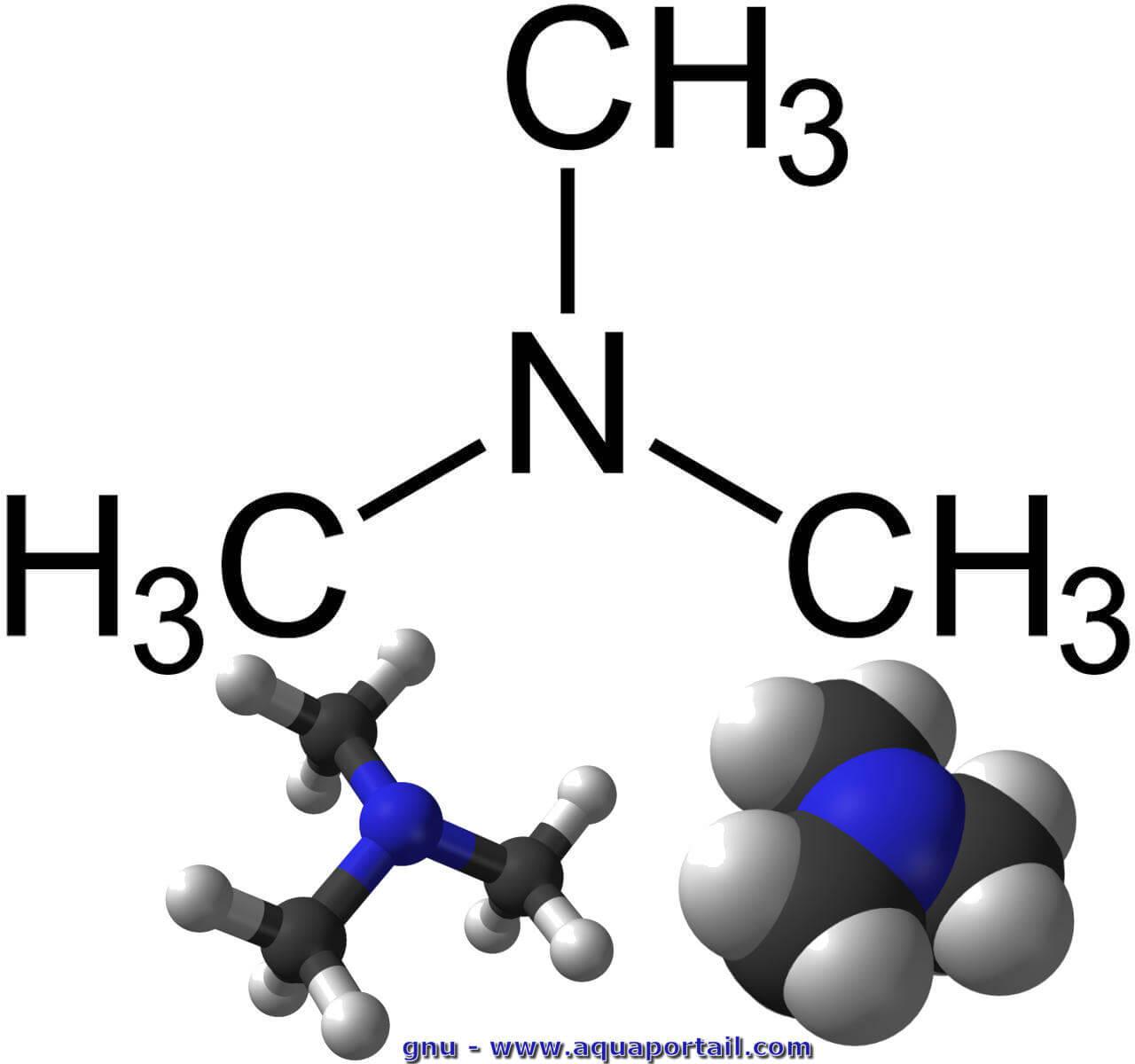
SOLVED: 'The compound trimethylamine, (CH3)3N, is a weak base when dissolved in water Write the Kb expression for the weak base equilibrium that occurs in an aqueous solution of trimethylamine: Kb'
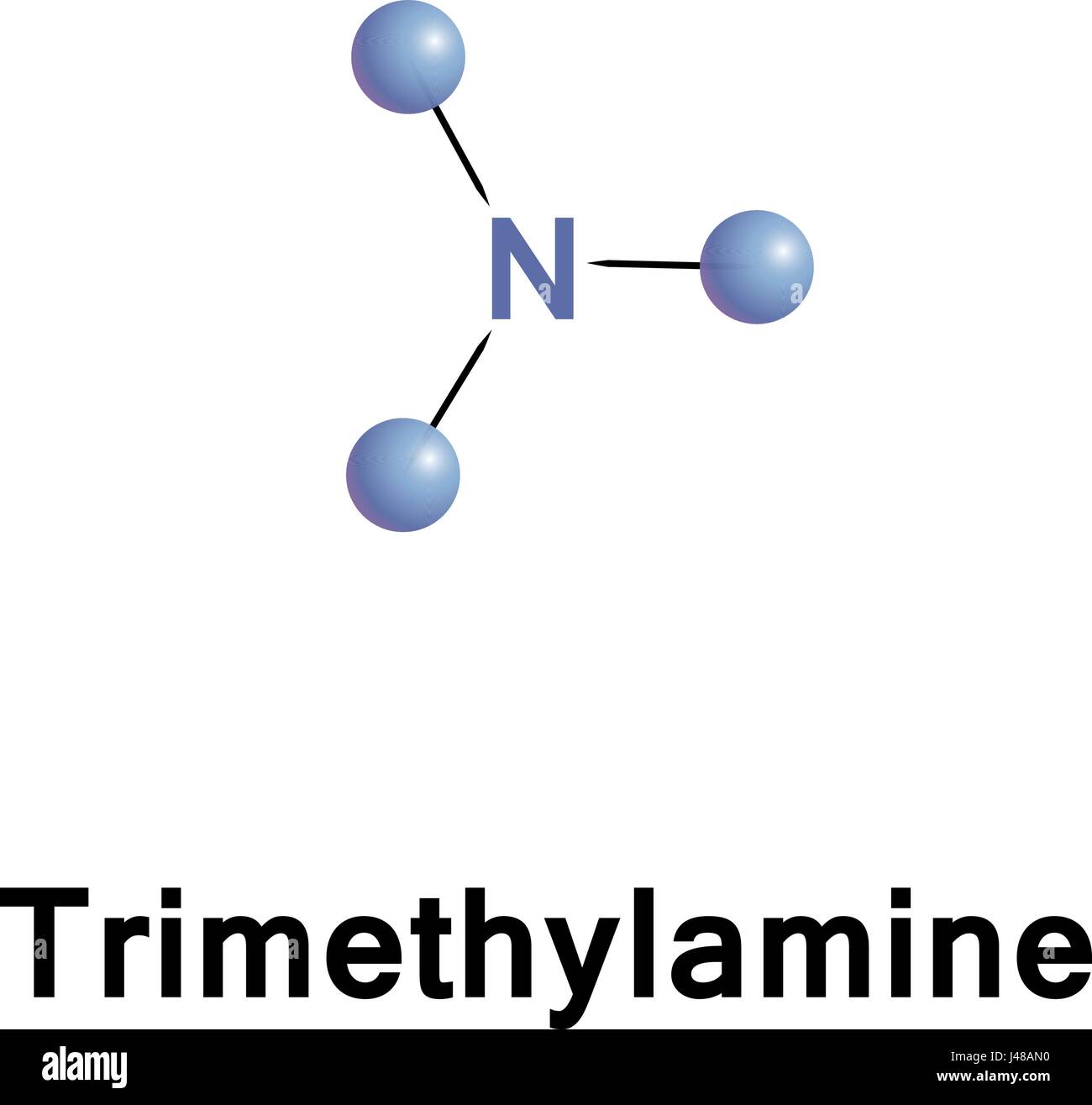
Trimethylamine is a nitrogenous base and can be protonated to trimethylammonium cation. This colorless, hygroscopic, and flammable tertiary amine has Stock Vector Image & Art - Alamy

Calculate the pH of a 0.443 M aqueous solution of trimethylamine. (Kb = 6.3 x 10-5) | Homework.Study.com
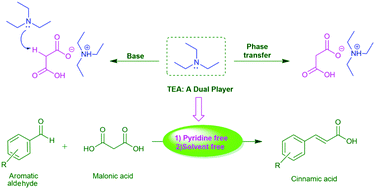
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing)