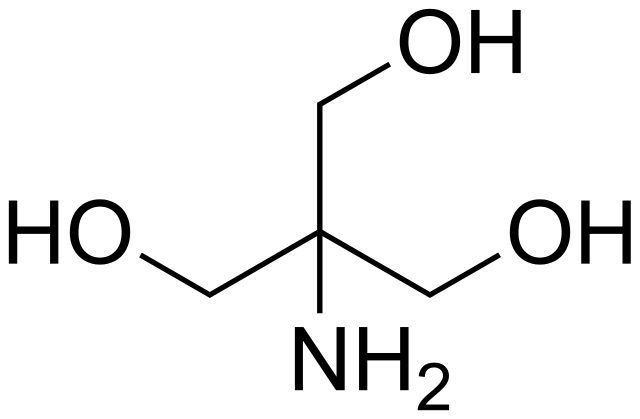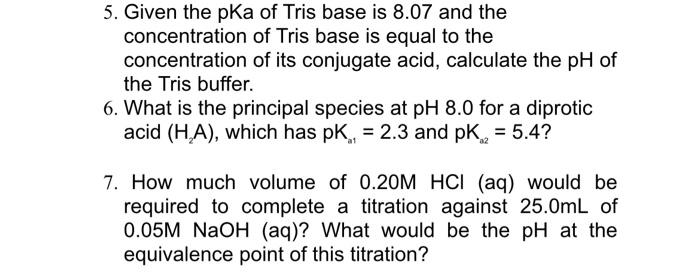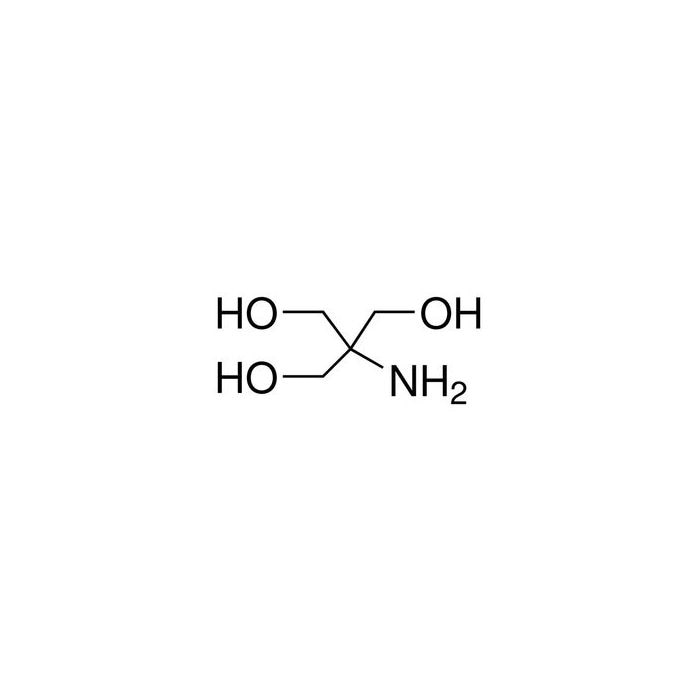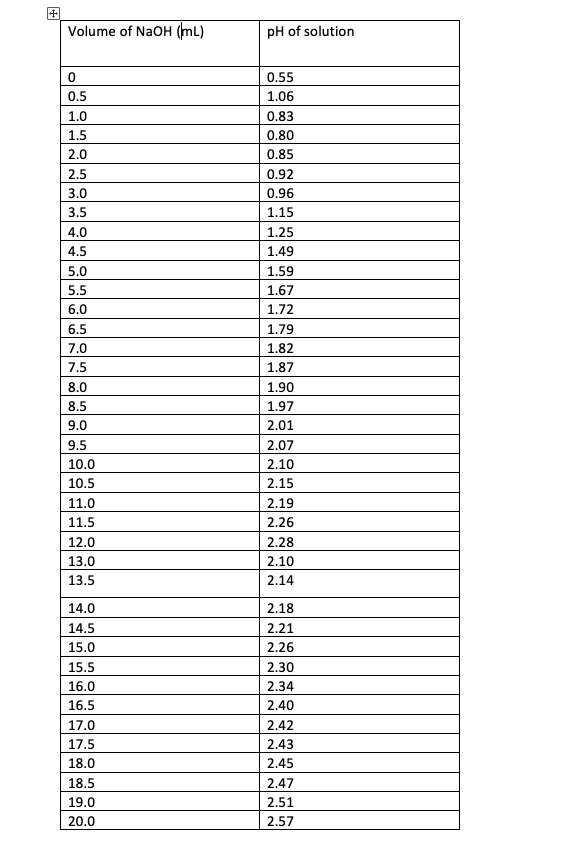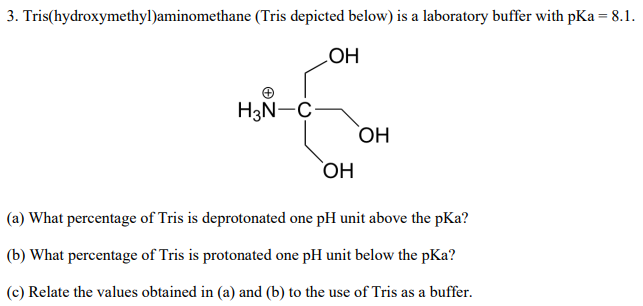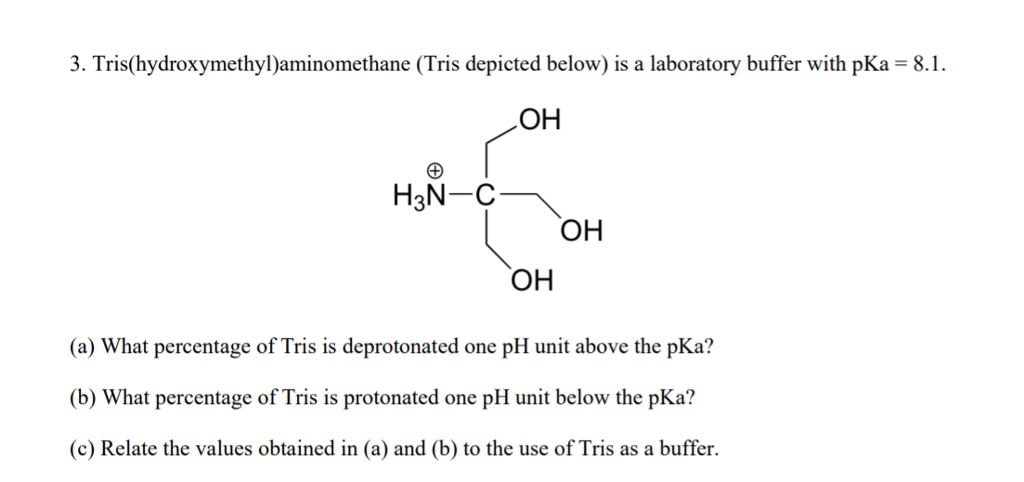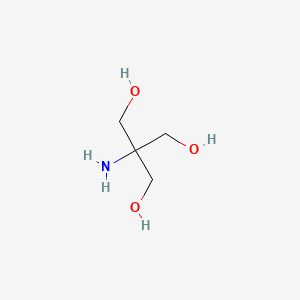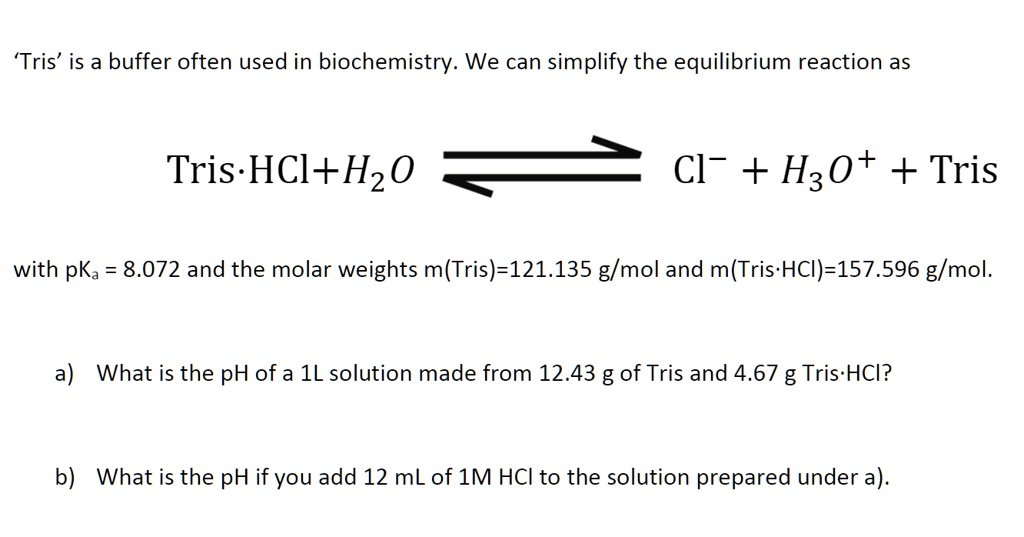
SOLVED: Tris' is a buffer often used in biochemistry. We can simplify the equilibrium reaction as: Tris-HCl + H2O -> Cl- + H3O+ + Tris with pKa = 8.072 and the molar

Interaction of Tris with DNA molecules and carboxylic groups on self-assembled monolayers of alkanethiols measured with surface plasmon resonance - ScienceDirect
![RPI TRIS Base Ultra Pure Powder, 1 Kilogram, Molecular Biology Grade, Buffer Component, [Tris (Hydroxymethyl) Aminomethane]: Amazon.com: Industrial & Scientific RPI TRIS Base Ultra Pure Powder, 1 Kilogram, Molecular Biology Grade, Buffer Component, [Tris (Hydroxymethyl) Aminomethane]: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/71xgmTB-JtL.jpg)
RPI TRIS Base Ultra Pure Powder, 1 Kilogram, Molecular Biology Grade, Buffer Component, [Tris (Hydroxymethyl) Aminomethane]: Amazon.com: Industrial & Scientific

Dissociation steps and pKa values at 25 °C and 37 °C of the buffers... | Download Scientific Diagram
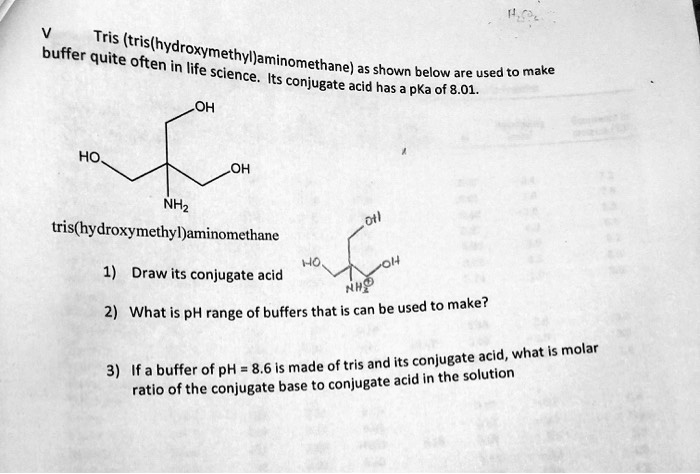
SOLVED: Tris buffer is quite often used in life science. Tris(hydroxymethyl)aminomethane is a common buffer that has a conjugate acid with a pKa of 8.01. The structure of tris(hydroxymethyl)aminomethane is shown below:
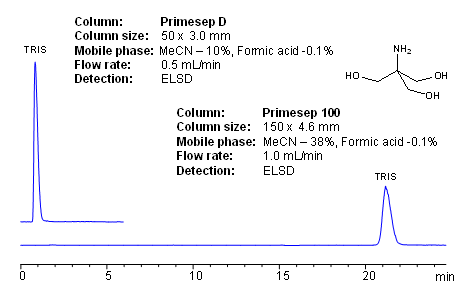
HPLC Method for Analysis of Trometamol (Tris, Tris(hydroxymethyl)aminomethane, Tromethamine, and or THAM) | SIELC Technologies